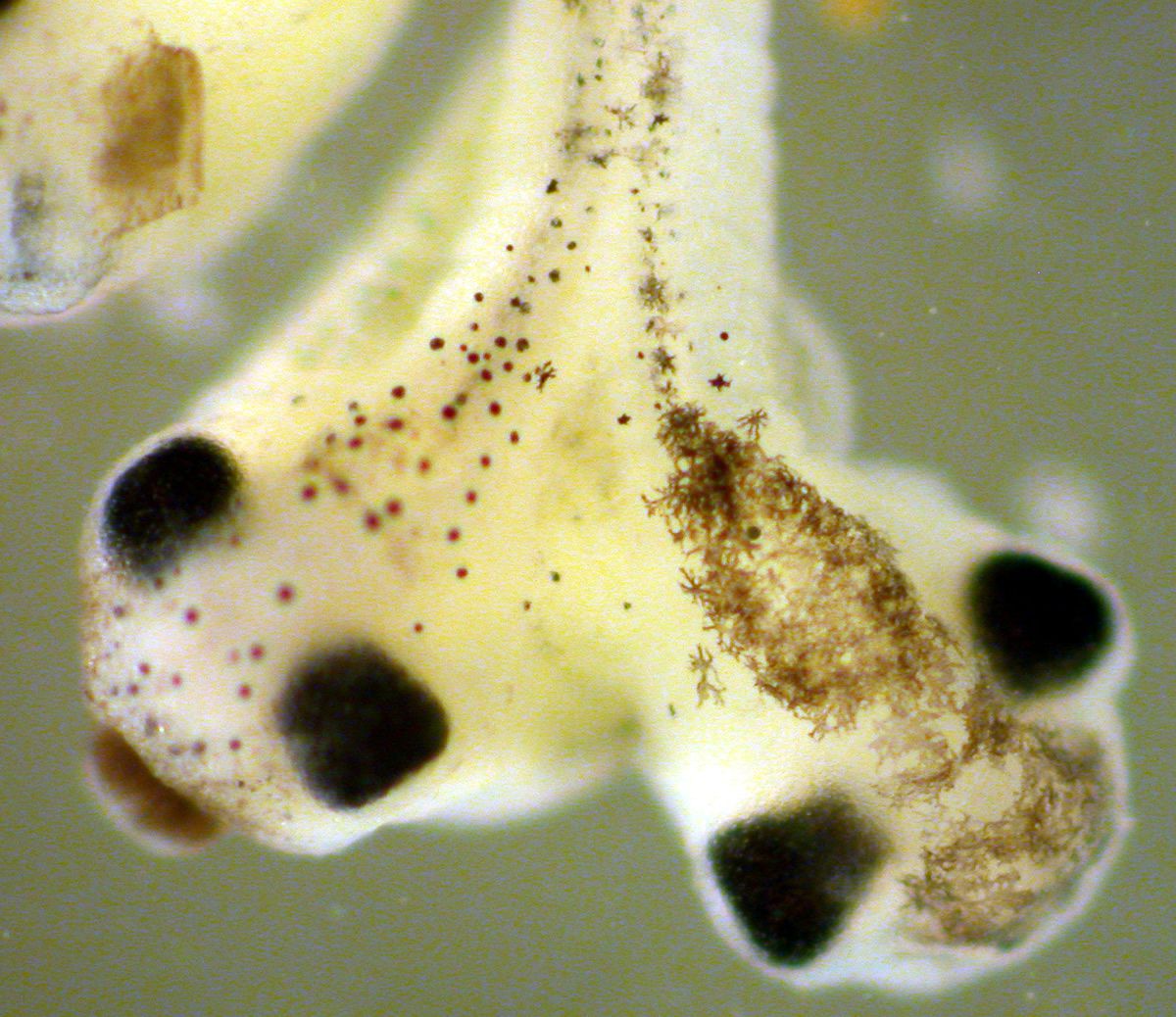
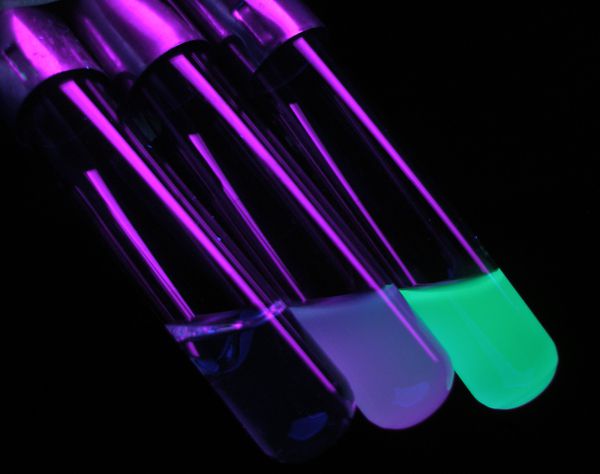
Photo Contest IV: Finalists, Lab Rats
We received approximately 30 photographs from 14 photographers. Most of the pictures were excellent. We divided the entries into 2 categories, Lab Rats and General, though we had to fudge a little bit to populate both categories.
Choosing finalists was difficult. We considered what we thought were the scientific and pictorial qualities of the photographs, and also attempted to represent as many photographers and present as much variety as possible. The text was written by the photographers and lightly edited for consistency.
Here are the finalists in the Lab Rats category. Please look through them before voting for your favorite. You will have to be logged in to vote on the poll. We know it is possible to game these polls. Please be responsible and vote only once. If we think that the results are invalid, we will cancel the contest. The photos and poll are below the fold.
The winner in each category will receive an autographed copy of Among the Creationists, by Jason Rosenhouse, which received a very favorable review here. We are indebted to the author for his generosity in providing the books.
Acknowledgement: Reed Cartwright wrote the HTML code.