Behe vs Sea Squirts
Recent research has thrown an interesting spanner into one of the key, but slightly obscure claims Behe makes about “irreducible complex” (IC) systems. In Behe’s discussion of the mammalian clotting system (Darwin’s Black Box [DBB], 1996, page 86, 1st edition) he claims:
“…none of the cascade proteins are used for anything other than the formation of a blood clot”.
This is a fundamental claim with important implications. If components of an allegedly IC system have other functions, this would violate his “well matched parts” condition for an IC system. Also, if these enzymes have other functions, they could be coopted from those functions to form a clotting system. If the clotting enzyme thrombin’s only function was to cut fibrinogen to make fibrin, then, if a mutation produced a thrombin-like enzyme in the absence of fibrin, natural selection would be unlikely to preserve this enzyme (but see below). On the other hand, if a general protease (an enzyme that cuts up lots of different proteins) were to gain the ability to break down fibrinogen, then its other functions would keep it preserved until a fibrinogen-like substrate appeared.
Contrary to Behe’s statement, many of the clotting proteins have other roles. Several of these non-clotting functions were known when Behe wrote DBB [1,2 and Note 1]. These roles, in wound healing and in tissue remodelling and embryogenesis, give us useful clues to their evolution. They also demolish Behe’s claims about IC.
A Myriad of Functions
Let’s have a look at some of these functions. But first a little background. The majority of enzymes in the clotting cascade are serine proteases, that is, enzymes that cut up proteins using the amino acid serine as the key catalytic group. The best-known serine protease is the enzyme trypsin, which helps us digest our food. Trypsin also plays important roles outside digestion as well, including responses to infection.
Molecular phylogeny suggests that trypsin and the clotting enzymes share a common ancestor [3]. Furthermore, the clotting enzymes prothrombin/thrombin, Factor X, Factor IX, Factor VII, and several others, all appear to be duplicates of each other [3,4].
Figure 1. Click for larger version. Gene duplication in the clotting system. Orange, duplicates of core serine proteases, Factors X, VII and IX are all believed to be derived from prothrombin, in turn derived from another serine protease. Light Blue: Factors VIII and V are duplicates of the ceruloplasmin family. Yellow, factor XIII is a member of the transglutamase family. Dark blue, Factor XI, is a duplicate of prekallikrein.
Serine proteases are ancient enzymes, present in unicellular eukaryotes, and have a wide range of physiological roles in digestion, defence and tissue remodelling. One of these roles involves activation of a class of cell surface proteins called proteinase activated receptors (PARs). Clotting enzymes, as befits serine proteases and relatives of trypsin, also act on PARs [1,5,6].
Activated Factor VII and Factor X both activate PAR2 [5,7] . This receptor is also activated by trypsin released by damaged epithelial cells, and other serine proteases released from mast cells and white blood cells during injury or inflammation [8]. Activated Factor X and thrombin (and trypsin) turn on PAR1, which amongst other things, activates neutrophils and causes aggregation of platelets [5,7,8]. In protovertebrates without a clotting system, wounds are plugged with haemocytes, primordial versions of the white blood cells and platelets that are activated by thrombin and trypsin. Trypsin or trypsin-like enzymes, leaking from damaged cells attracting haemocytes to plug a wound, would be the start of a protoclotting system.
As well as activating PARs, thrombin also appears to be an alternative activation enzyme for the complement pathway, which is involved in passive immunity [9]. It is important to note that activation of white blood cells and complement systems predates the appearance of the clotting system. Indeed, in protovertebrates the prime function of the complement system is to produce masses of sticky protein to tangle up bacteria, making this an excellent candidate for a protoclotting system [10].
Continuing on this theme, the clotting factor XIII, which cross-links the loose fibrin clot into a hard clot, is also involved in wound healing, tissue remodelling and the formation of new blood vessels [11,12]. Factor XIII does this via clotting-independent mechanisms [11,12]. It can cross-link a number of connective proteins, for example integrin, which allows inflammatory and wound-repair cells to migrate more easily to damaged sites. Factor XIII also binds to some growth factor receptors, and results in tissue growth [11,12]. Interestingly, while in mammalian clotting, factor XIII needs to be activated by thrombin, a form of XIII is found in platelets and special white blood cells (macrophages and neutrophils) that can be activated and released independently of thrombin [11]. This active form of Factor XIII is found circulating in the blood of fish (mammals have no circulating active Factor XIII of any kind) [12]. This form of factor XIII is also found sea squirts, invertebrate relatives of ours, in the cells that form haemocytes [13]. Sea squirts don’t have the specialized blood cells that we do. They utilise a couple of generalized blood cell types, called haemocytes, more on this later.
So let’s briefly recap: several clotting factors, VII, X, Thrombin and XIII – in fact, the major components of the extrinsic clotting system, the system that is primarily responsible for forming clots – have other functions. And not minor functions – the tissue remodelling functions, for example, are particularly important.
But Does it Matter?
Now, you may say, “So What, Behe got his statement wrong, but it doesn’t invalidate his argument about IC and blood clotting, does it? After all, it’s just one throwaway sentence in a whole chapter.”
But you would be wrong. This is a key point on which Behe’s argument turns. The fact that these enzymes have other actions means that they could be doing another job, and then be co-opted into a clotting role. The irreducibly complex pentachlorophenol degradation pathway was produced by mutation and recruitment of a single enzyme. Behe says that if a protothrombin developed before fibrinogen, then it would be functionless, an enzyme “twiddling its thumbs” (DBB, p. 95) that would be removed by natural selection before a fibrinogen appears. But a protothrombin that is activating a PAR receptor has a job to do: attracting haemocytes to a wound to plug it. Remember that trypsin-like proteins are released when there is tissue damage, so a protothrombin derived from trypsin or a related enzyme would be released in the vicinity of haemocytes when a protovertebrate is cut. If a mutation to this protothrombin fortuitously made it able to cleave a protein that then clumped together, this then forms the basis of a protoclotting system. The addition of a globbing protein, even a weak one, to the haemocyte patch would inevitably make it stronger and more stable, offering a selective advantage [Note 2].
Waiting for Fibrinogen
In case you think this is a bit far fetched, sea squirts have a thrombin-like serine protease that can convert mammalian fibrinogen to fibrin, making a clot [14]. But sea squirts don’t have fibrinogen [15], in fact they don’t clot their heamolymph (their equivalent of blood) either, they just plug all cuts with a loose haemocyte patch. So the thrombin-like protein must be doing something different, yet it is capable of acting like thrombin. So much for Behe’s “thumb twiddling”.
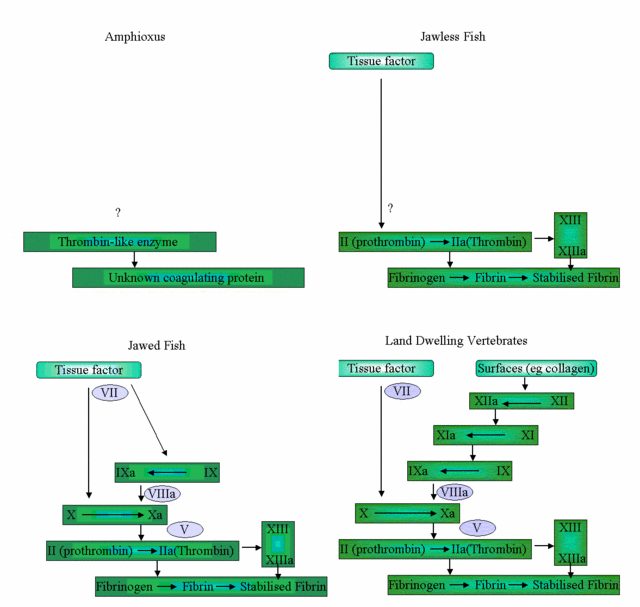
Figure 2. Click for a larger version. The clotting systems of various chordates. Fish lack the intrinsic coagulation system, Jawless fish lack Factor X and the cephalochordates lack pretty much everything except a thrombin-like protein, but they can still clot their haemolymph. Sea squirts (not shown), have a thrombin-like protein but don’t clot their haemolymph.
As I’ve noted before, some of the complement enzymes have thrombin-like properties, and as they are also released during inflammation, they could be recruited to form a clotting system. Also, while protovertebrates such as sea squirts have no true fibrinogen, their haemocytes are enriched with the protein cortical granule lectin [16]. This protein has fibrinogen domains which could become a substrate for a protothrombin. Cortical granule lectin and similar proteins with a fibrinogen domain in sea squirts appear to be involved in passive immunity as proteins that makes bacteria “sticky”, which makes them ideally preadapted for becoming a part of a protoclotting system.
The chordate amphioxus (in modern taxonomy, Branchiostoma and its relatives), a protovertebrate that looks a bit like a minature eel, does clot its haemolymph. It has a thrombin-like enzyme (we don’t know if it is a true thrombin yet, but it clots mammalian plasma just like mammalian thrombin), but no true fibrinogen [4]. Clearly, Behe’s claim that all the enzymes of the clotting pathway must be in place to form a useable clot is incorrect.
A Path is revealed
Inspection of the non-clotting roles of the clotting enzymes also suggest how a clotting system could be assembled piecemeal. Bear with me for a moment, as this is going to get technical.
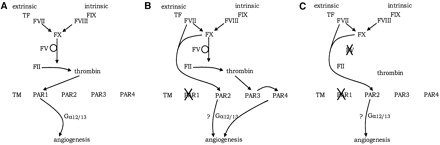
In developing embryos, development of blood vessels (angiogenesis) depends on thrombin activating PAR-1 [5]. However, you can knock out the thrombin pathway and the blood vessels will still form. Factor VII and Factor X can substitute for thrombin [5,6]. Thus a smaller, simpler version of the clotting system can work doing a non-clotting job of activating PARs, and this system could be built up into a full system acting on PARs by duplication and mutation with neofunctionalisation, and then mutations which allowed the protothrombin to act on a protofibrinogen would have produced a workable primitive clotting system.
Figure 3. Despite knocking out thrombin, Factor X and Factor VI activate angiogenesis by alternate pathways. Figure taken from [5]
Another possible, but simpler, pathway suggests itself. Invertebrates such as lobsters and shrimp have a simple “one step” coagulation system, where a Factor XIII-like clotting factor is released by haemocytes and crosslinks a coagulation protein into a clot. Sea squirts have a short Factor XIII protein [13], which can be activated and released from haemocytes via non-standard pathways. As sea squirts do not clot their haemolymph, the most likely role of the short Factor XIII is in wound repair, as it is in vertebrates, where it crosslinks and stabilises tissue matrix proteins [11,12]. While the short Factor XIII is able to be activated in the absence of thrombin, thrombin will also activate the short Factor XIII [11]. As tissue damage in sea squirts also releases trypsins and trypsin-like proteins (and there is the thrombin like serine protease already mentioned [14]), it is not too difficult for a mutation on one of these tryspin-like enzymes to result in a trypsin/proto-thrombin that activates short Factor XIII, making it more effective at cross-linking and stabilizing wounds. As there is no feedback amplification in this system, the activated factor XIII is effectively restricted to the wound site. This is not dissimilar to the trypsin/prophenyloxidase system, in amphioxus, where a trypsin activates an enzyme (prophenyloxidase) to make gluggy melanin at wound sites [17].
A simple mutation to the small Factor XIII would allow it to crosslink one of the protofibrinogens, such as cortical granule lectin, that are present in, and released from, haemocytes. This would make a simple, if weak, clot that could stabilise the haemocytes. Yet another mutation in the protothrombin could make the protofibrinogen bind its substrate as well – the protofibrinogens are sticky proteins anyway (and are often used as a glugging factor in innate immunity, making bacteria sticky so they stick to each other or stick to phagocytes [16,18]). So the production of a self-associating protein that could be stabilised by further by the small factor XIII is highly probable.
Summary and take home Message
Now, I’ve presented three plausible scenarios above: (1) protothrombin as a haemocyte attractant, (2) protothrombin as a part of the complement system, and (3) prothrombin as an activator of Factor XIII. All of them are consistent with what we know of existing coagulation systems in vertebrates and invertebrates. All of them are potentially testable via molecular clocks and whole-genome studies on a variety of protoverebrates (we need the amphioxus genome, apparently due out this year) and vertebrates, as well as reconstruction of the common ancestral proteins.
However, Behe and other ID apologists will undoubtedly dismiss these scenarios as not detailed enough. This misses the point. The point is that Behe has claimed that his argument showed in principle that the coagulation system could not evolve from simpler systems. The simple fact that both sea squirts and amphioxus have thrombin-like enzymes, but no true fibrinogen (and, in the case of sea squirts, any other of the clotting components), demolishes Behe’s argument. Having evidence for the intermediate steps in the evolution of the clotting cascade is just the icing on the cake. Behe and others can complain all they like about the details, but the take-home message is that “irreducible complexity,” as described by Behe, is no barrier to evolution of complex systems.
Notes
Note 1: While Behe is an exemplary structural biochemist/biophysicist, he does get the biology a bit off centre, sometimes to amusing effect. In his discussion of the clotting system, he spends a long time detailing the labyrinthine intrinsic clotting system as an exemplar of “ICness”. Neither fish nor whales have the complete intrinsic clotting system, and they do just fine.
Note 2: Even in mice, which have a significant dependence on clotting factors, if you knock out fibrinogen, platelet (the mammalian equivalent of haemocytes) aggregation in full-thickness skin incisions will stop the mouse from bleeding to death. You can see how adding even minor amounts of sticky proteins to the plug would be beneficial.
Another note: Nick Matzke corrected various typos in the original posting.
-
- Reuning U, Little SP, Dixon EP, Bang NU. Effect of thrombin, the thrombin receptor activation peptide, and other mitogens on vascular smooth muscle cell urokinase receptor mRNA levels. Blood. 1994 Dec 1;84(11):3700-8
-
- Tsopanoglou NE, Pipili-Synetos E, Maragoudakis ME. Thrombin promotes angiogenesis by a mechanism independent of fibrin formation. Am J Physiol. 1993 May;264(5 Pt 1):C1302-7
-
- Doolittle RF. The evolution of vertebrate blood coagulation: a case of Yin and Yang. Thromb Haemost. 1993 Jul 1;70(1):24-8.
-
- Davidson CJ, Tuddenham EG, McVey JH. 450 million years of hemostasis. J Thromb Haemost. 2003 Jul;1(7):1487-94.
-
- Moser M, Patterson C. Thrombin and vascular development: a sticky subject. Arterioscler Thromb Vasc Biol. 2003 Jun 1;23(6):922-30.
-
- Major CD, Santulli RJ, Derian CK, Andrade-Gordon P. Extracellular mediators in atherosclerosis and thrombosis: lessons from thrombin receptor knockout mice. Arterioscler Thromb Vasc Biol. 2003 Jun 1;23(6):931-9.
-
- Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004 Jun;24(6):1015-22.
-
- Steinhoff M, et al., Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005 Feb;26(1):1-43.
-
- Huber-Lang M, et al., Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006 Jun;12(6):682-7.
- 10.Krem MM, Di Cera E. Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem Sci. 2002 Feb;27(2):67-74.
-
- Adany R, Bardos H. Factor XIII subunit A as an intracellular transglutaminase. Cell Mol Life Sci. 2003 Jun;60(6):1049-60.
-
- Monsonego A, et al., Factor XIIIa as a nerve-associated transglutaminase. FASEB J. 1998 Sep;12(12):1163-71.
-
- Cariello L, Ristoratore F, Zanetti L. A new transglutaminase-like from the ascidian Ciona intestinalis. FEBS Lett. 1997 May 19;408(2):171-6.
-
- Shishikura F, Abe T, Ohtake S Tanaka K Purification and characterization of a 39,000-Da serine proteinase from the hemolymph of a solitary ascidian, Halocynthia roretzi Comp Biochem Physiol B Biochem Mol Biol. 1997 Sep;118(1):131-41.
-
- Jiang Y, Doolittle RF. The evolution of vertebrate blood coagulation as viewed from a comparison of puffer fish and sea squirt genomes. Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7527-32.
-
- Lee JK, Baum LG, Moremen K, Pierce M. The X-lectins: a new family with homology to the Xenopus laevis oocyte lectin XL-35. Glycoconj J. 2004;21(8-9):443-50.
-
- Pang Q, Zhang S, Wang C, Shi X, Sun Y. Presence of prophenoloxidase in the humoral fluid of amphioxus Branchiostoma belcheri tsingtauense. Fish Shellfish Immunol. 2004 Nov;17(5):477-87.
-
- Kenjo A, et al., Cloning and characterization of novel ficolins from the solitary ascidian, Halocynthia roretzi. J Biol Chem. 2001 Jun 8;276(23):19959-65.